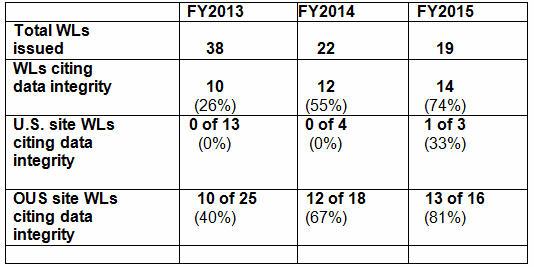
Starting Early
In phase I, researchers are testing a new drug or treatment in a small group of volunteers for the first time to evaluate its safety, to determine a safe dosage range and to identify side effects. The amount of drug product manufactured is relatively small and easy to control at this point in the product’s development.
But when a clinical trial progresses, the formulation itself and the dosage form may change as factors like stability become better characterized. As a result, sponsors may also want to consider the desirable commercial format in later stage clinical studies
Patient and clinic needs will also change as the drug travels from small phase I trials to large multi-center phase III trials.
For flexibility around dosage it is often best to use a granule dosage form in capsules, and the dose can then be adjusted simply by changing the weight or number given.
Catalent conducts stability trials of the study drug alongside the clinical trial and will likely be testing different primary packaging configurations such as solid dose tablets or capsules in blister packs and bottles as part of that process.
Stability data will influence the pack design, and so the dosage form used at this stage may bear little resemblance to the final commercial form.
Packaging is also based on the volume of anticipated patient recruitment. Randomization of patients to treatment types can be stratified to test dosing of patients by gender, age, body weight and other factors. From a complexity point of view, the pack designs themselves don’t tend to be complex.
“What does get more complex after phase I are factors like randomizations and labeling because the customer and Catalent are trying to develop the best dosage form for patients. Stratified randomizations may be used when testing characteristics such as body weight, gender, age alongside dosage strength. As a result, the randomizations tend to be quite complex due to the number of variables involved ,” said Steve McMahon, process leader at Catalent.
Most often, supplies are sent to the clinical site rather than directly to patients, and labeling might be more complex because labels will include a unique number that links back to the randomization and how a patient is being dosed, McMahon said. Clinical sites usually manage inventory in-house due to shorter turn-around times, and so their ability to store items might also come into play.
More becomes known about the study drug as the trial progresses to phase II trials, where the drug is given to a larger group of patients to test for the right dosage and further evaluate its safety.
Based on stability data and other feedback, pack design and materials may change to a more appropriate dosage form, such as tablets, extended-release capsules, infusions or injections.
“If a long expiry date is not possible, then you might try and simplify the pack design,” he said, noting that in phase II, more stability data will be collected in a rolling stability trial, in which case expiry dates are extended as more data are captured.
“There are lots of different factors that we’ll start with, depending on the expiry date,” McMahon said. “All the time you have to be mindful about what the customer wants to do in phase III and what is required when you commercialize the product.”
Some packaging protects stability better
One of the best forms of primary packaging to protect the product and improve the stability of a product is cold form blister packets. Materials used have a polyvinyl chloride (PVC) or polyvinylidene chloride (PVDC) seal, which is dense and resistant to temperature and humidity.
“The good thing about blistering is that only one tablet is popped at a time, so the stability of the rest of the drug product is maintained,” McMahon stressed. “Whereas, when using a bottle, once the bottle is opened, the entire pack is exposed to humidity.”
Storage conditions need to be considered in the dosage form and pack design he said, noting that a site could be storing a product at -80˚C while the trial is ongoing, but the product might be distributed at -20˚C.
These types of decisions surrounding the dosage form can also affect turn-around times for trial sites.
“For example, Catalent had a customer for a relatively large-scale phase II trial, and were doing packaging runs about every three months. Catalent did the initial primary packaging and then secondary packaging. Then every three months the customer received more stability data and Catalent was required to re-label packaged supplies with new expiry dates.”
“From a customer point of view this was costing them more money.”
He explains that the practice of putting expiry dates on clinical trial packaging is required in some jurisdictions. In the U.S., expiry dates are not compulsory on packaging for clinical trials because they can be controlled by interactive technology [IRT]. Expiry date information is also sent to the site.
The trial medication is managed through an automated pre-determined order system based on initial order and resupply parameters in the IRT system. Since the IRT manages expiry dates, it makes the whole supply chain a lot smoother. It also helps control costs due to less risk of over producing patient kits which may expire before they can be dispensed.
But in Europe, even if an IRT is used, the clinical trial packaging still needs to be labeled with expiry dates, he noted.
“It’s important to have strict standard operating procedures of how to do these things, and Catalent has extensive experience in doing multi-pack designs and labeling,” McMahon stressed.
“Even though it can be complex, we will produce batch documents that detail exactly what we need to do to for quality reviews internally and for the client, and after execution of that production run, it gets reviewed again. The controls in place for clinical trials are more restrictive than in commercial drug manufacturing, because the controls include management of blinded materials.”
By the time a drug gets to phase III trials, where it will be given to larger groups of patients often multi-country or global to confirm its effectiveness, a significant increase in production is quite common.
Companies will also start running multi-center studies in multiple countries, and production could shift from 250 units at phase I to two million units or more at phase III.
Ensuring patient compliance
Packaging and labeling in phase III are developed with regulatory and legal requirements, patient and sponsor needs as well as commercial requirements in mind, and the study may be randomized and blinded against a placebo or more likely a market leading comparator. Clinical trial packaging will often move to wallets or cards with blister packs or individual bottles that are sent to the clinical sites, which then distribute them to patients who generally self-administer at home.
In phase I, the drug is administered at a specialized Phase I clinical site that has dispensing pharmacists with GMP training who can make-up dose capsules or dispense tablets from a bottle. This translates into strict patient compliance. But when patients start taking the study drug at home, this is when compliance can start to slip. One solution is to use packaging designs that make it easier for the patient to comply with the protocol dosing schedule, such as sun and moon symbols on blister cards to indicate AM vs. PM doses.
“One of the big things that put patients off taking medication is non- patient centric packaging and labeling,” McMahon stressed. “If they don't understand the pack or the labeling or it's cumbersome, they may not take the drug.”
Why outsource clinical supplies?
McMahon said that most pharmaceutical companies are geared up for mass manufacturing, and they don't really have the scale and capability to do smaller scale clinical runs.
Some companies have separate units for clinical trials, but that often ends up costing more in the long run to keep such a unit up and running.
“It's beneficial for big pharmaceutical companies to use integrated providers like Catalent because we've got the experience and expertise of doing clinical trials, and we have the equipment to handle most packaging designs and clinical trial packaging. Some customers might have a high potency or controlled drug and their facility might not be set up for that.”
There are several factors that biopharmaceutical companies should take into account when vetting a drug delivery solution provider such as Catalent or contract manufacturer (CMO) for outsourcing primary packaging. Some of these vendor considerations include:
- The ability of a vendor to expand packaging capacity on short notice. This may be due to constraints on storage, labor or equipment capacity at the customer’s own sites;
- Taking advantage of a vendors experience/specialty in a particular packaging field such as blister packaging of solid dose forms;
- Reduced risk to drug development timelines due to vendor’s’s experience in packaging and distribution;
- Major cost savings in not having to incur capital expenditure on specialized equipment, recruitment and training of staff;
- Vendors can help reduce drug development time with different packaging strategies;
- A vendor’s geographical presence of packaging and distribution centers can provide logistical savings;
- The vendor will usually have more flexible production timelines with the capability to manage milestones on a project critical path; and
- Some vendors have the necessary experience and contacts to procure comparator products from multiple sources.
McMahon said the quality unit and the Qualified Person (In Europe) at a company will be able to advise if a planned packaging process or pack design is compliant with Regulatory Authorities such as the FDA, MHRA good manufacturing practices regulations or country specific requirements.
The customer quality unit would also typically audit and approve the CMO before placing work.
The CMO’s packaging design group can also confidentially leverage their collective experience across a variety of projects to help current customers devise their optimal packaging solution. . This guidance can take the form of best practices or lessons learned that the customer may not have been aware of on their own.
The CMO can provide feedback on whether the pack design is suitable for packaging and distribution to clinical sites and patients across the world. It can also inform on whether the pack is the correct size to match the various phases of the protocol and the dispensing visits.
Costs can also be managed by consulting with the CMO, because the components used to make a pack design can increase or decrease in cost but can also add value to a patient through ease of use. This would give increased probability of patient compliance to the trial.
Investigator and clinical staff, as well as the patient can also provide valuable feedback on the pack design, particularly about whether the product is easy to dose and if labeling instructions are easily followed.
“We have a patient-centric initiative at Catalent with the aim of putting the patient first. Part of this initiative resulted in some of our pre-production people visiting a clinical site to get feedback,” McMahon said, noting that staff are often surprised how small the clinical site can be and why storage might be a problem. Alternatively, a doctor or a nurse in a large hospital will want packaging that stands out and very clear labelling because they could be running multiple trials, and they need to be able to identify the right clinical supplies quickly.
Make primary packaging decisions early
To accelerate drug development, companies need to consider clinical trial packaging strategies as early as possible in the development process.
“There are several factors that need to be considered from the customer’s point of view, such as whether they have the capacity and resources for the packaging for a trial. If the answer is no, then the customer has to start thinking about outsourcing straightaway,” he said.
Some customers will have capacity for a phase I trial or a small phase II trial, but then a CMO will be needed to handle Phase III. For a smooth transition, companies need to plan for this early to leave room for auditing the CMO to make sure they have the necessary quality standards. Formal agreements also need to be put in place and disclosure that outsourcing is going to occur should be included in any trial documentation.
“That is not a quick turnaround, and it could take up to six months to prepare, which is a long time during that critical stage,” McMahon advised.
Companies should also consider the CMO’s packaging and distribution network. “For example, what if a trial needs to be conducted in Australia, what are you going to do? Does the customer require trial material to be shipped from the UK? If it's a temperature-sensitive product, I don't think that's necessarily a good idea. Working with a CMO with an extensive distribution network and the right expertise can give you other options that may be more advisable.”
He said that Catalent has global manufacturing capability across six continents, and can seamlessly switch gears to meet clinical trial needs, so shipping to remote regions in Australia wouldn’t be a problem.
Another challenge for drug companies can be sourcing a comparator product, because innovator companies may put up road blocks to sourcing their material until they are assured the product will be used within an appropriate setting and not subject to parallel trade as they are legally responsible for that drug.
“This can either be a high mark up on the original cost of the drug, quantity or lot limits, an extremely long lead time on it or difficulty in obtaining the necessary paperwork,” McMahon said. Catalent has a specialized team with well-established relationships with innovators (preferred route) and wholesalers to expedite the sourcing and purchasing of comparators.
The complexity of some study designs in clinical trial packaging can also create challenges, and the different types of clinical supply models that a customer may follow can have a major impact on the packaging strategy.
The traditional clinical supply model, called supply-led packaging, is a centralized stock-based approach that uses discrete primary and secondary packaging runs to bulk-ship finished patient kits to clinical sites and depots based on estimated demand.
Under this model, primary and secondary packaging is undertaken at centralized GMP packaging facilities where stock is built up well in advance of actual need. This model can cause an under- or over supply due to variations in patient recruitment rates, which can end up being quite wasteful or carry a greater risk of supply not meeting demand.
Just-in-time labeling
The just-in-time labeling model uses discrete primary and secondary packaging runs the same as in the traditional model to produce base-labeled patient kits that are held within a central physical inventory to await final labeling.
Under this scenario, packaging is accomplished via large-batch runs. This model is efficient when the study involves materials that are not likely to require expiry update management. Or, it could a good option when the expiry date is very short and the product is on a concurrent stability program, and the most up-to-date expiry date can be added at the point of shipment.
“A potential issue is the customer still has all these bottles produced, so you could still be producing too much product. What does give you the flexibility is when recruitment is even across different countries.”
However, labeling at the time of shipment can result in longer associated lead times and result in potential bottle necks. In addition, more quality resources are needed.
Yet another option is multi-language booklets that have individual country-specific instructions inside, and then that carton can be sent anywhere around the world.
Demand-led supply model
The demand-led supply (DLS) model is a dynamic, continuous GMP approach to secondary packaging, labeling, release and distribution.
Under this model, made-to-order patient kits are shipped to clinical sites from regional facilities based on actual patient demand. Secondary packaging takes place at a regional, full-service packaging facility where a supply of unlabeled but uniquely coded ‘bright stock’ is placed in advance. Accordingly, the primary packaging plan should take into consideration the lead time required to ship bright stock from the central primary packaging facility into the regional packaging facilities. By using bright stock, not only can the exact kits needed be made-to-order, but drug product can potentially be pooled for use across multiple protocols which can potential reduce the amount of drug that goes unused..
Then, based on forecasted demand, this bright stock is distributed to regional GMP facilities where it awaits further processing. Secondary packaging is completed and the finished patient kit is shipped to the investigator site where it is needed within a matter of days in response to on-demand orders received via IRT.
The advantage of this model is that secondary orders are fulfilled based on what is actually needed by the sites, leading to more efficient use of stock, significantly reduced risk of stock-outs and virtually eliminating the need to update expiry labeling at the investigator site.
Since labeling is not completed until just before the material is sent to the site, expiry dates can reflect the most current stability data available. This translates into minimal drug waste and can be a major cost savings for some products.
One future enhancement for labeling is 2D bar codes. Some will even have hyperlinks inside the bar codes that enable the user to scan them with a smart phone to get country-specific instructions.
The demand-led model is also a good choice when a comparator drug is needed and is difficult to source, very expensive, or in short supply. This model is also well suited for orphan drug products and certain specialty products that are often subject to extreme limited availability and very tight distribution control.
Quality and due diligence
The approach to good manufacturing practices should be the same regardless of the phase of a clinical trial. It is critical that quality personnel are involved in the development of a study from the start to ensure compliance to GMP at all stages.
The complexity of a study means greater involvement and understanding of quality personnel. “Compliance to GMP, the Orange Guide, FDA regulations for labeling and packaging prevents issues from arising,” he said.
“Spending more time upfront in planning, taking care to use established standard operating procedures and best practices produces greater efficiencies and reduced errors,” McMahon stressed.
And, if the complexity of a study requires a deviation from an SOP, then this needs to be documented pre-production appropriately.
Compliance ensures faster approvals for label artwork, batch documentation and ultimately faster availability to the patient. That translates into fewer delays in production and ultimately clinical trial data that is accepted by regulators.
For inspecting primary packaging, Catalent uses cameras to inspect the blister packs after they've been filled, or the camera can also be set up before the packs are sealed as well.
The primary packaging materials themselves can be challenging, because certain materials are more difficult to work with. For cold form blister packing, the actual pocket is formed by pressure, and the material basically bends into a pocket.
When it comes to high potency drug products, many CMOs have specialized packaging suites that are used exclusively for these types of drugs because they typically can’t be packaged in normal primary packaging rooms. Pharmaceutical companies that do not typically have potent and cytotoxic drugs in their portfolios may find that they do not have the necessary infrastructure in place to package these drugs on their own.